Congress Materials – American College of Gastroenterology (ACG 2024)
2024 American College of Gastroenterology | Oct 25-30 | Philadelphia, PA
This section includes selected Johnson & Johnson Innovative Medicine abstracts, posters, or oral presentations, which have been accepted for congress presentations in the last 2 years or less (as determined by any congress restrictions on length of time materials can be posted). Information about pipeline products or investigational uses of products does not imply FDA approval for these products or uses, nor does it establish the safety or efficacy of these products or uses. Johnson & Johnson does not recommend or suggest use of its medicines in a manner inconsistent with FDA-approved labeling. The content contained in this section is subject to congress copyright permissions.
Corticosteroid-Sparing Effects of Treatment with Guselkumab in Patients with Moderate to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results Through Week 44
Brian Bressler, Jessica R. Allegretti, David T. Rubin, Nicole Shipitofsky, Kuan-Hsiang G. Huang, Matthew Germinaro, Rebbecca Wilson, Hongyan Zhang, Alessandro Armuzzi, Sigal Fishman, Yufang Wang, Julián Panés, Gary R. Lichtenstein, and Laurent Peyrin-Biroulet
Efficacy and Safety of Guselkumab Maintenance Therapy Among Guselkumab Induction Week 24 Clinical Responders: Results From the Phase 3 QUASAR Maintenance Study
David T. Rubin, Axel Dignass, Jessica R. Allegretti, Shadi Yarandi, Kuan-Hsiang G. Huang, Matthew Germinaro, Joyce Zhan, Hongyan Zhang, Yaser Rayyan, Masayuki Saruta, Domingo Balderramo, Brian Bressler, Laurent Peyrin-Biroulet, Tadakazu Hisamatsu
Efficacy and Safety of Subcutaneous Guselkumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results Through Week 48 From the Phase 3 GRAVITI Study
Remo Panaccione, Ailsa Hart, Flavio Steinwurz, Silvio Danese, Tadakazu Hisamatsu, Qian Cao, Mobolaji Olurinde, Marion L. Vetter, Zijiang Yang, Yuhua Wang, Jewel Johanns, Natalie A. Terry, Bruce E. Sands, Geert D’Haens
Efficacy and safety of subcutaneous guselkumab rescue therapy in patients with moderately to severely active Crohn’s disease and inadequate response to ustekinumab: phase 2 GALAXI 1 study long-term extension results
A. Afzali, D. Wolf, R. Leong, L. Salese, L. Gao, C. Busse, J. Panés
Efficacy of Guselkumab versus Placebo in Crohn’s Disease Based On Prior Response/Exposure to Biologic Therapy: Results of the GALAXI 2 & 3 Phase 3 Studies
Bruce E. Sands, Geert D’Haens, Silvio Danese, Tadakazu Hisamatsu, Walter Reinisch, Natalie A. Terry, Leonardo Salese, Rian Van Rampelbergh, Zijiang Yang, Jewel Johanns, George DuVall, Niazy Abu Farsakh, Remo Panaccione
Efficacy of Guselkumab in Moderately to Severely Active Crohn’s Disease According to Induction Clinical Response Status: Week 48 Results from the GALAXI 2 & 3 Phase 3 Trials
Anita Afzali, Julián Panés, Bruce E. Sands, David T. Rubin, Remo Panaccione, Natalie A. Terry, Leonardo Salese, Rian Van Rampelbergh, Zijiang Yang, Mary Ellen Frustaci, Dino Tarabar, Minhu Chen, Emese Mihaly, Douglas Wolf, Geert D’Haens
Guselkumab Binding to CD64+ IL-23–producing Myeloid Cells Enhances Potency for Neutralizing IL-23 Signaling
Jessica R. Allegretti, Raja Atreya, Maria T. Abreu, Amy Hart, He (Hurley) Li, Tom C. Freeman, Eilyn Lacy, Matthew DuPrie, Phuc Bao, Tina Smets, Joshua Wertheimer, Indra Sarabia, Kristen Kohler, Anne Fourie, Kacey Sachen
Guselkumab Decreases Key Cellular Inflammatory Processes Across Ileum and Colon Tissue in Crohn’s Disease
Dylan Richards, Swati Venkat, Darren Ruane, Martha Zeeman, Natalie A. Terry, Marion Vetter, Mario Gomez, Daniel Cua, Tom C. Freeman, Bradford McRae, Brian Feagan, Walter Reinisch, Patrick Branigan
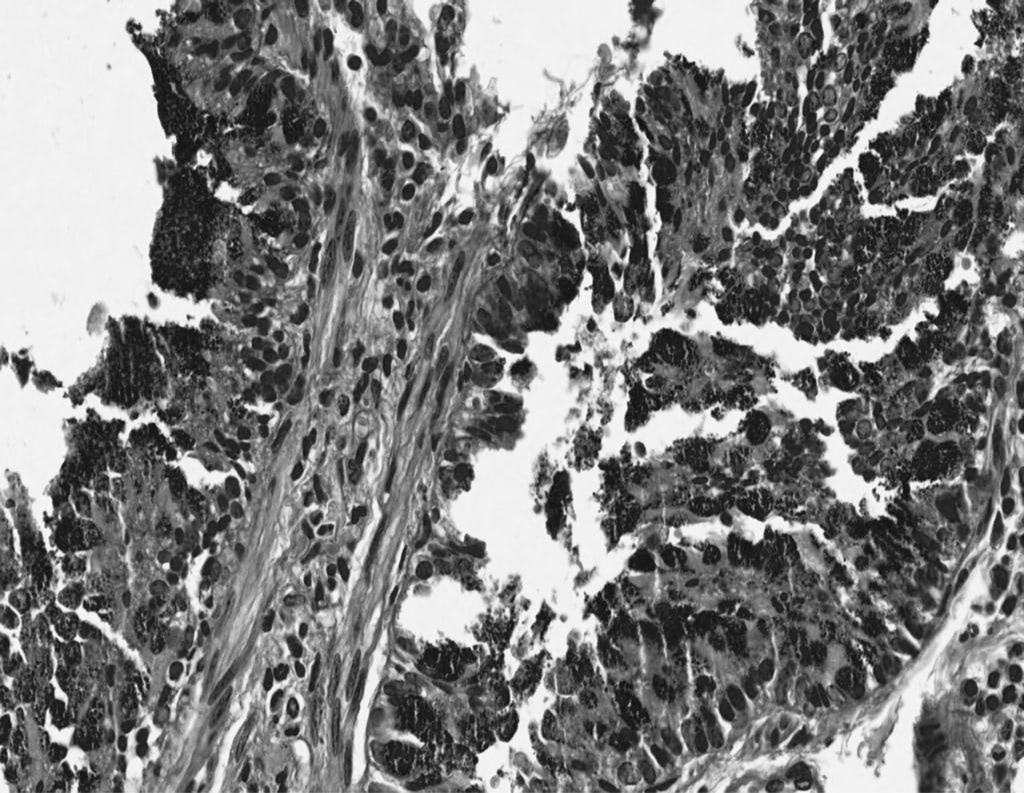
Histologic and Combined Histologic and Endoscopic Outcomes After Guselkumab Maintenance Therapy in Patients With Moderately to Severely Active Ulcerative Colitis: Week 44 Results From the Phase 3 QUASAR Maintenance Study
J. Panés, A. Dignass, T. Hisamatsu, S. Yarandi, K.G. Huang, M. Germinaro, S. Sridhar, P. Branigan, R. Wilson, H. Zhang, F. Magro, V. Jairath, B.G. Feagan, G.R. Lichtenstein, D.T. Rubin, B.E. Sands
IBD Patient Preference Study Comparing Monotherapy and Dual Biologic Therapy Among Primary and Secondary Non-responders
Ellen Janssen, Anna Sheahan, John Lynch, Melissa Marko, Matthew Wallace, Meena Bewtra, Jui-Chen Yang, Reed Johnson, Laura M Bozzi
The Efficacy of Maintenance Treatment with Guselkumab in Patients with Moderately to Severely Active Ulcerative Colitis: Phase 3 QUASAR Maintenance Study Results at Week 44 by Biologic/Janus Kinase Inhibitor History
J.R. Allegretti, J. Panés, L. Peyrin-Biroulet, B.E. Sands, S. Yarandi, K.-H.G. Huang, M. Germinaro, J. Zhan, H. Zhang, J. Begun, J. Kierkuś, T. Kravchenko, T. Hisamatsu, D.T. Rubin, B. Bressler, and A. Dignass
Week 48 Efficacy of Guselkumab and Ustekinumab in Crohn’s Disease Based On Prior Response/Exposure to Biologic Therapy: Results from the GALAXI 2 & 3 Phase 3 Studies
Silvio Danese, Anita Afzali, Remo Panaccione, Julián Panés, Walter Reinisch, Natalie A. Terry, Leonardo Salese, Rian Van Rampelbergh, Kitty Yuen Yi Wan, Zijiang Yang, Jewel Johanns, Marcin Zmudziński, Eran Zittan, Katsuyoshi Matsuoka, Vipul Jairath, David T. Rubin
Copies of these presentations are for personal use only and may not be reproduced without written permission from the presentation author.